Pfizer and BioNTech have surprised the world, and given it hope, with the preliminary results of the Phase 3 clinical trial of their coronavirus vaccine.
They announced on Nov. 9 that the early analysis of the data from the Phase 3 clinical trial, which is still ongoing, showed the vaccine was 90 per cent effective. The unexpectedly high figure still needs to be confirmed in larger numbers and over time. The vaccine is simple to manufacture, but its storage is more complex (the vaccine must be kept at very low temperatures). Large-scale production is expected to begin shortly.
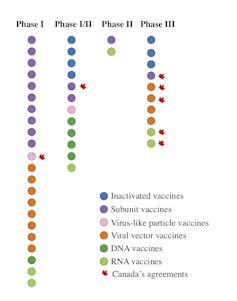
Laboratories around the world are in a race to produce a COVID-19 vaccine. While the finish line is now in sight for Pfizer and BioNTech, the World Health Organization (WHO) lists 202 vaccine candidates, 47 of which are in human trials.
Canada has not put all its eggs in one basket in its plan to protect Canadians from the novel coronavirus. In addition to Pfizer and BioNTech, it has signed six other contracts: Moderna, Sanofi and GlaxoSmithKline, Johnson & Johnson, Novavax, AstraZeneca and Medicago. Canada recently announced it had reserved 56 million additional doses of vaccine from Pfizer and BioNTech, on top of the 20 million doses it had already purchased, bringing its order to 76 million.
Canada has secured access to a total of 414 million doses of COVID-19 vaccines from different sources. More importantly, Canada has ensured that it has diversified the types of vaccines it will have.
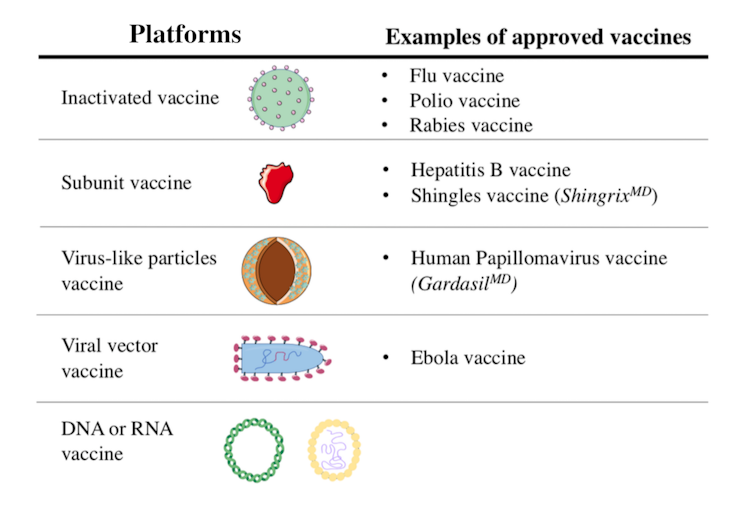
Scientists are using different platforms to develop COVID-19 vaccines. Some vaccine candidates in clinical trials exploit mechanisms already used in other vaccines. Others are based on innovative approaches that have never been tested before. Here is an overview of the different types of vaccines.
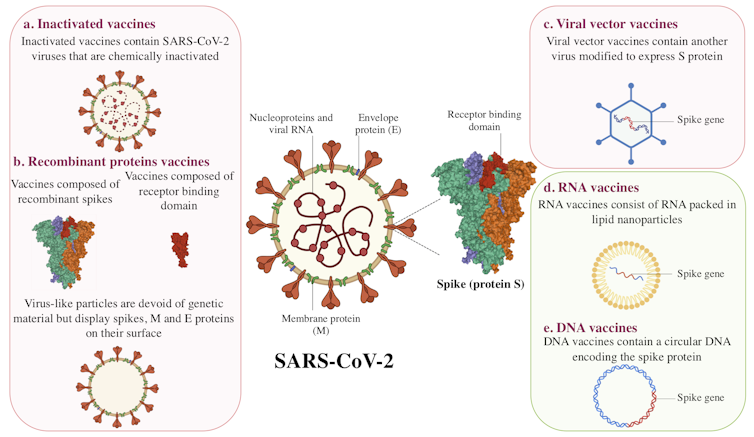
Inactivated vaccines
Inactivated vaccines have been in use since the 1880s. The viruses in these vaccines have been rendered inactive by chemical treatment, as with SARS-CoV-2 candidate vaccines, or by physical treatment.
With this type of vaccine, the immune system encounters the virus in its entirety. It can mount a defence when it detects the viral spike protein (also called spicule or S-protein), envelope and nucleoprotein.
Currently, seven inactivated vaccine candidates are being tested in humans. Of these, three are in Phase 3 clinical trials. Unlike Phase 1 and Phase 2, which are used to evaluate a vaccine’s tolerability, safety and ability to induce an immune response, a Phase 3 clinical trial allows scientists to test its efficacy.
Recombinant proteins
Recombinant protein vaccines fall into two categories: subunit and virus-like particle vaccines.
For subunit protein vaccines, a viral protein is produced in large quantities in a living “factory,” such as a bacterium, plant, mammalian or insect cell. When the viral protein is presented to the immune system, it triggers a reaction.
The 13 subunit vaccine candidates currently in Phase 1, 2 or 3 clinical trials are composed of either the entire spike protein or a specific portion of the spike protein called the ‘receptor binding domain’.
Virus-like particle vaccines are composed of a set of viral proteins that mimic the shape of the virus. This particle “pseudo-virus” is an empty shell, devoid of genetic material and non-infectious, but this does not prevent the immune system from recognizing it.
Viral vector vaccines
This approach is based on using a virus that is non-pathogenic or of little danger to humans. In the case of the 12 vaccine candidates of this type currently being studied in humans, the viral vectors are mostly adenoviruses. They represent a large group of viruses that can cause colds and conjunctivitis, among other symptoms.
Used as Trojan horses, these viruses are modified to express the SARS-CoV-2 spike protein following vaccination. Viral vector vaccines are a recent strategy, but were used in the development of the Ebola virus vaccine.
RNA and DNA vaccines
Despite differences in their composition, DNA and mRNA (messenger RNA) both contain genetic information for protein production. While an RNA molecule can directly produce that information, DNA requires an intermediate transcription step.
RNA or DNA vaccine candidates differ from other strategies in two ways. First, it is a novel strategy: there is no RNA or DNA vaccine on the market. Second, they are the only vaccine candidates composed solely of genetic material.
In the case of RNA vaccines, messenger RNA molecules are wrapped in lipid nanoparticles. Once the vaccine is injected, the RNA serves as a template for the body’s cells to produce a viral protein — the spike protein, to be precise.
DNA vaccines, on the other hand, are made up of a circular DNA (called a plasmid). This DNA will be transcribed into RNA, which will again serve as a template.
Six RNA vaccine candidates are currently being tested in humans, two of which are in Phase 3. The five DNA vaccine candidates are in Phase 1 and 2 clinical trials.
Canada in the vaccine race
The following is an overview of each company that has an agreement with the federal government, the type of vaccine in development and the number of doses reserved by Canada.
Pfizer/BioNTech: 76 million doses reserved
BioNTech, in Germany, and Pfizer, in the United States, are jointly developing an RNA vaccine. This candidate encodes for the manufacture of the spike protein.
The Phase 3 clinical study is continuing with more than 43,000 patients in the U.S., Argentina, Brazil, Germany, Turkey and South Africa. The vaccine is reported to be more than 90 per cent effective and has not caused any serious side-effects.
Despite these encouraging preliminary results, Pfizer and BioNTech have not yet crossed the finish line. Detailed data have not been published and questions remain, including the age and risk factors of the vaccinated individuals and the duration of protection. The clinical trial is ongoing and more data will be analyzed.
Moderna: 56 million doses reserved
The vaccine candidate of Moderna, a U.S.-based biotechnology company, is an RNA vaccine. Once injected, it allows the expression of the spike protein. Currently in Phase 3 clinical trials, the vaccine is being tested in 30,000 individuals in different regions across the United States.
Johnson & Johnson: 38 million doses reserved
Johnson & Johnson’s candidate is a viral vector vaccine composed of a human adenovirus that has been modified to render it incapable of multiplying, but capable of expressing the SARS-CoV-2 spike protein. The Phase 3 clinical trial, which began in September 2020, is taking place in several countries and will involve 60,000 participants.
AstraZeneca/University of Oxford: 20 million doses reserved
Oxford University is partnering with AstraZeneca on a viral vector vaccine. The vaccine candidate is composed of a modified chimpanzee adenovirus that expresses the spike protein. It is in Phase 3 clinical trials.
Novavax: 76 million doses reserved
The vaccine candidate of Novavax, a U.S. company, is based on the recombinant protein strategy. It is composed of the spike protein and an adjuvant, a booster used in vaccines to increase the immune response. The Phase 3 clinical trial began in September 2020 and involves 10,000 participants in the United Kingdom.
Sanofi/GSK: 72 million doses reserved
The candidate from the French company Sanofi and the British giant GlaxoSmithKline (GSK) is composed of an adjuvant and recombinant version of the spike protein, produced in a living factory (baculoviruses). Phase 1 and 2 clinical trials are currently testing its safety, tolerability and ability to induce an immune response.
Medicago: 76 million doses reserved
Developed by the Québec-based company Medicago, this vaccine candidate is composed of virus-like particles. These are produced in plants infected with bacteria that have been genetically modified to produce several SARS-CoV-2 proteins. These plants thus become production plants.
Researchers can extract the particles from the leaves and purify them. Medicago’s vaccine candidate is currently in Phase 1 clinical trials and results are also promising.![]()
Aïssatou Aïcha Sow, Doctorante en virologie moléculaire, Institut national de la recherche scientifique (INRS)
This article is republished from The Conversation under a Creative Commons license. Read the original article.